Catalysts that work quickly with maximum energy efficiently are the key to producing green fuels and one Korean university believes it has made a major step forward in the process transforming water to hydrogen for fuel
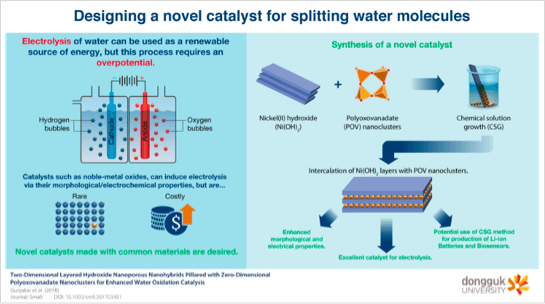
Globally, the transition to greener sources of energy requires the use of efficient catalysts for fuel generation reactions. Now, scientists at Dongguk University in South Korea have synthesised an efficient catalyst for the oxygen evolution reaction — a component of water-splitting process that produces hydrogen and oxygen.
The catalyst, synthesised using molybdenum and ruthenium, exhibits “high activity, reaction rates, and durability, opening doors to the cost-effective and large-scale production of next-generation catalysts using diverse materials”.
“Chemical energy storage is a promising solution for circumventing the global energy challenges,” says a statement put out by university researchers. “Reduction of water to molecular hydrogen via the splitting water reaction is a key method. However, issues like low catalyst activity, slow reaction speed, and catalyst degradation pose challenges.”
Now, in a new study led by Professor Young-Kyu Han and Assistant Professor Jitendra N. Tiwari from the Department of Energy and Materials Engineering at Dongguk University, scientists have developed an effective oxygen evolution reaction catalyst with molybdenum and ruthenium. Their study was made available online on July 29, 2023, and published in Volume 339 of Applied Catalysis B: Environmental on December 15, 2023. According to Prof. Han, “Carbon materials are crucial for commercial acidic polymer electrolyte membrane water electrolyzers. At high voltages, however, carbon atoms degrade in strongly acidic media, necessitating the need for new catalyst materials beyond carbon materials.”
This latest announcement follows at least four years of research by the university and there are other scientific teams around the world working to discover the perfect catalyst material combination that offers low energy use to produce the maximum hydrogen from water. But the discovery that adding ruthenium oxide to the catalyst is recognised as a useful step forward.
The latest study gets a but technical for ordinary folk: it involves implanting ruthenium oxide into a two-dimensional molybdenum carbide to create a catalyst (Mo2TiC2Tx MXene) with high mass activity, turnover frequency, and durability. Calculations also indicated that the ruthenium sites had a strong affinity towards oxygen species, which enhanced the reaction.
These findings are significant, as the global aim is to achieve 80% renewable electricity by 2050. Hydrogen and oxygen have diverse industrial applications, spanning clean fuel, power generation, chemical production, and life-support systems. Notably, more than 90% of hydrogen is in petroleum recovery and refining (47%) and ammonia production (45%) alone.
The transportation sector will also benefit. Elaborating on this Dr Tiwari said: “The need for decarbonizing the transportation sector makes hydrogen a promising alternative. Going ahead, fuel cell vehicles are expected to efficiently convert hydrogen into electrical energy, emitting only water, with longer driving ranges than battery electric vehicles. Additionally, hydrogen fuel cells do not need recharging and don’t degrade if hydrogen fuel is present, unlike in batteries,” observes Dr. Tiwari.
This study thus serves as a guide for researchers to create new catalysts for acidic water oxidation. It also sheds light on achieving cost-effective, large-scale catalyst production using diverse materials, such as dual-transition metal catalysts.
Reference
Title of original paper: Atomic layers of ruthenium oxide coupled with Mo2TiC2Tx MXene for exceptionally high catalytic activity toward water oxidation





